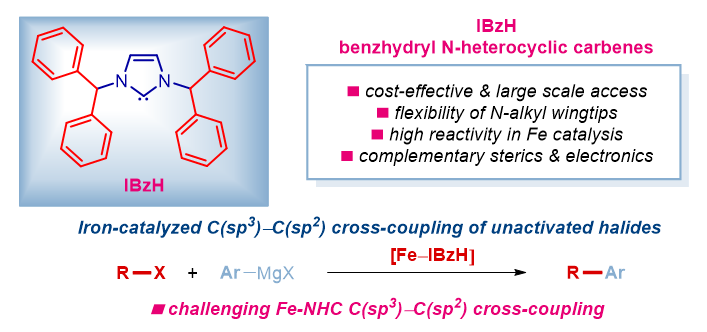
35) IBzH (IBenzhydryl): sterically-flexible N-aliphatic N-heterocyclic carbenes (NHCs) for iron-catalyzed C(sp3)–C(sp2) cross-coupling of unactivated haloalkanes
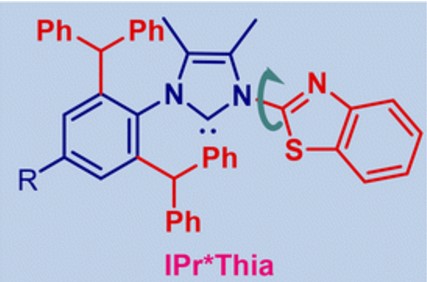
34) IPr*Thia – wingtip-flexible, sterically hindered, modular, N,C/S,C-chelating thiazole-donor N-heterocyclic carbene ligands
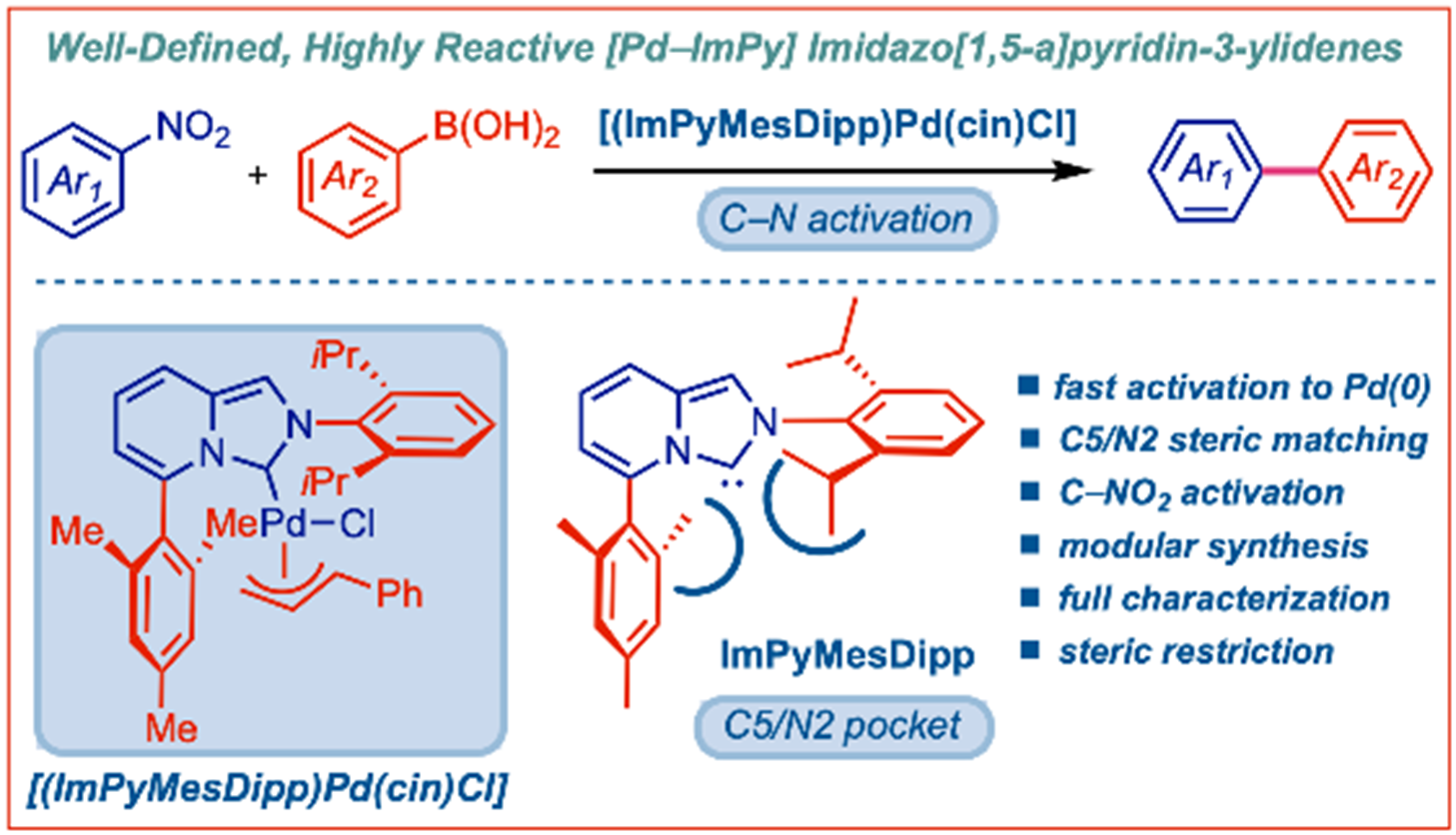
33) L-Shaped heterobidentate imidazo[1,5-a]pyridin-3-ylidene (N,C)-ligands for oxidant-free AuI/AuIII catalysis
P. Gao, J. Xu, T. Zhou, Y. Liu, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak, D. Zhang, M. Szostak*
Angew. Chem. Int. Ed. 2023, 62, e202218427

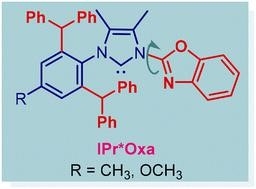
32) IPr*Oxa - a new class of sterically-hindered, wingtip-flexible N,C-chelating oxazole-donor N-heterocyclic carbene ligands
P. Podchorodecka, B. Dziuk, R. Szostak, M. Szostak, E. Bisz*
Dalton Trans. 2023, 52, 13608-13617
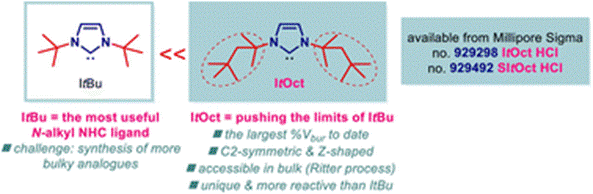
31) ItOct (ItOctyl) - pushing the limits of ItBu: highly hindered electron-rich N-aliphatic N-heterocyclic carbenes
M. M. Rahman, G. Meng, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak, M. Szostak*
Chem. Sci. 2023, 14, 5141-5147
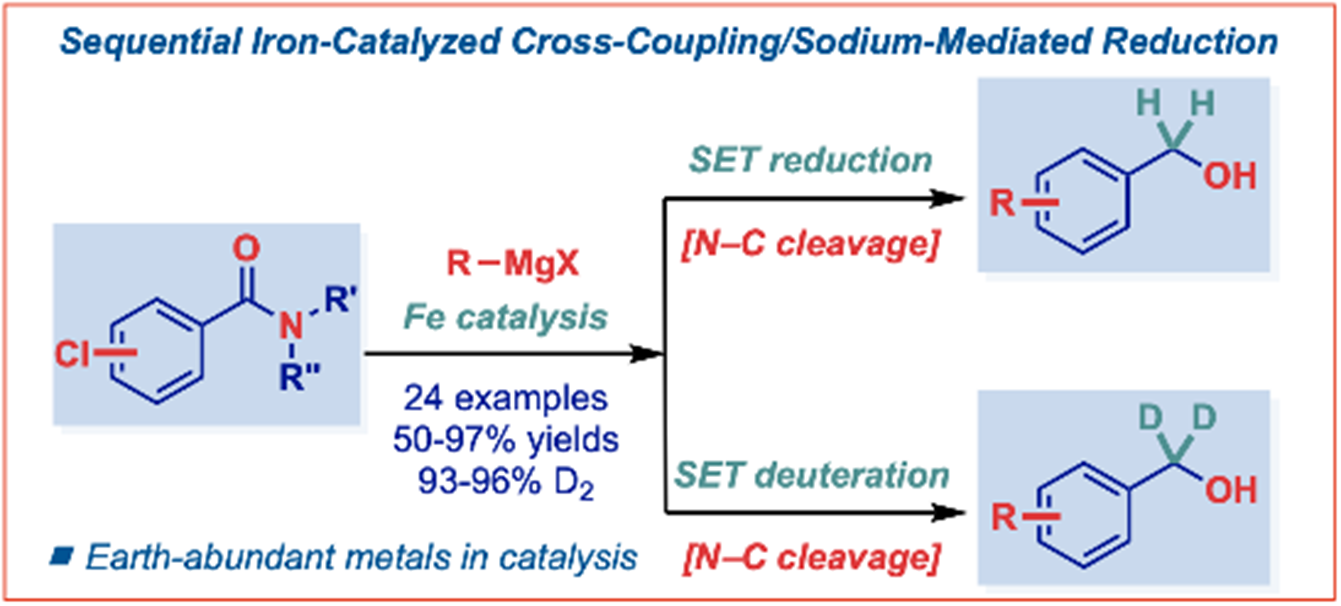
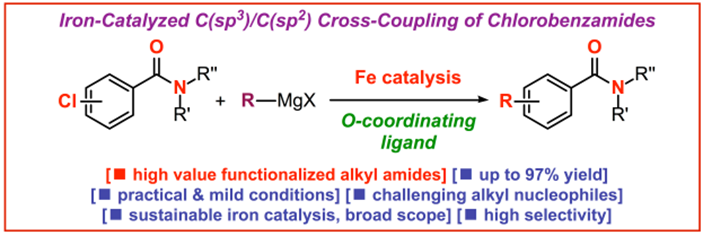
30) Sequential iron-catalyzed C(sp2)–C(sp3) cross-coupling of chlorobenzamides/chemoselective amide reduction and reductive deuteration to benzylic alcohols
E. Bisz*, P. Podchorodecka, H. Li, W. Ochędzan-Siodłak, J. An*, M. Szostak*
Molecules 2023, 28, 223-230
(Special Issue: Metal-Induced Molecule Activation and Coupling Reactions; Open-access: https://doi.org/10.3390/molecules28010223)
29) Pd-PEPPSI N-heterocyclic carbene complexes from caffeine: Application in Suzuki, Heck, and Sonogashira reactions
M. M. Rahman, J. Zhang, Q. Zhao, J. Feliciano, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak, M. Szostak*
Organometallics 2022, 41, 2281-2290

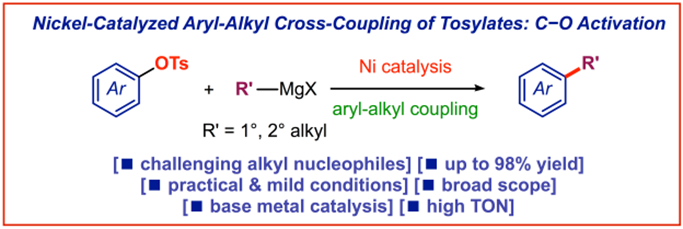
28) Enhanced activity of bulky N-heterocyclic carbenes in nickel-NHC catalyzed Kumada-Corriu cross-coupling of aryl tosylates
M. Kardela, K. Halikowska-Tarasek, M. Szostak, E. Bisz*
Cat. Sci. Tech. 2022, 12, 7275-7280

27) CAAC-IPr*: easily accessible, highly sterically-hindered cyclic (alkyl)(amino)carbenes
W. Chu, T.Zhou, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak, M. Szostak*
Chem. Commun. 2022, 58, 13467-13470

26) Well-defined, air- and moisture-stable palladium-imidazo[1,5-a]pyridin-3-ylidene complexes: a versatile catalyst platform for cross-coupling reactions by L-shaped NHC ligands
T. Zhou, P. Gao, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak, M. Szostak*
Cat. Sci. Tech. 2022, 12, 6581-6589
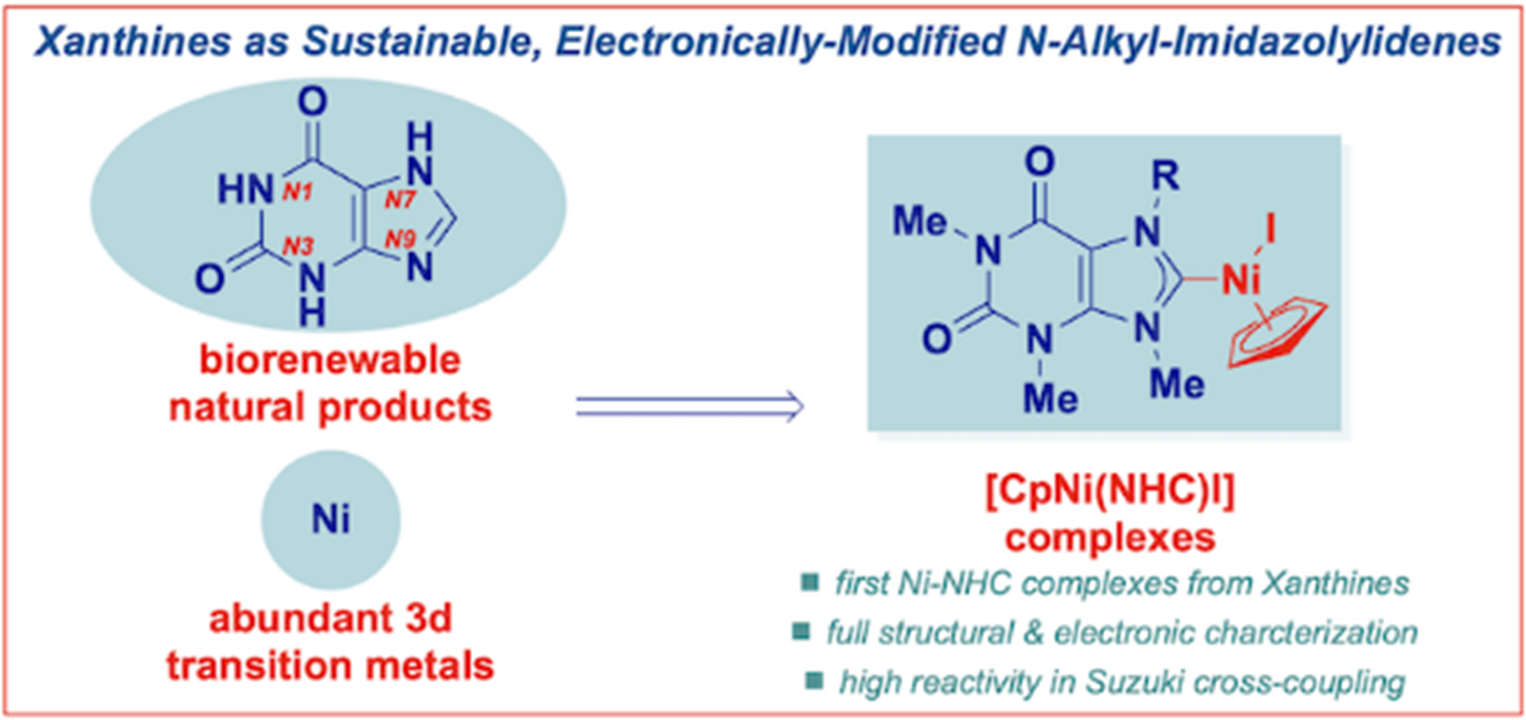
25) N-heterocyclic carbene complexes of nickel(II) from caffeine and theophylline: sustainable alternative to imidazol-2-ylidenes
J. Zhang, M. M. Rahman, Q. Zhao, J. Feliciano, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak, M. Szostak*
Organometallics 2022, 41, 1806-1815
(Special Issue: Sustainable Chemistry)
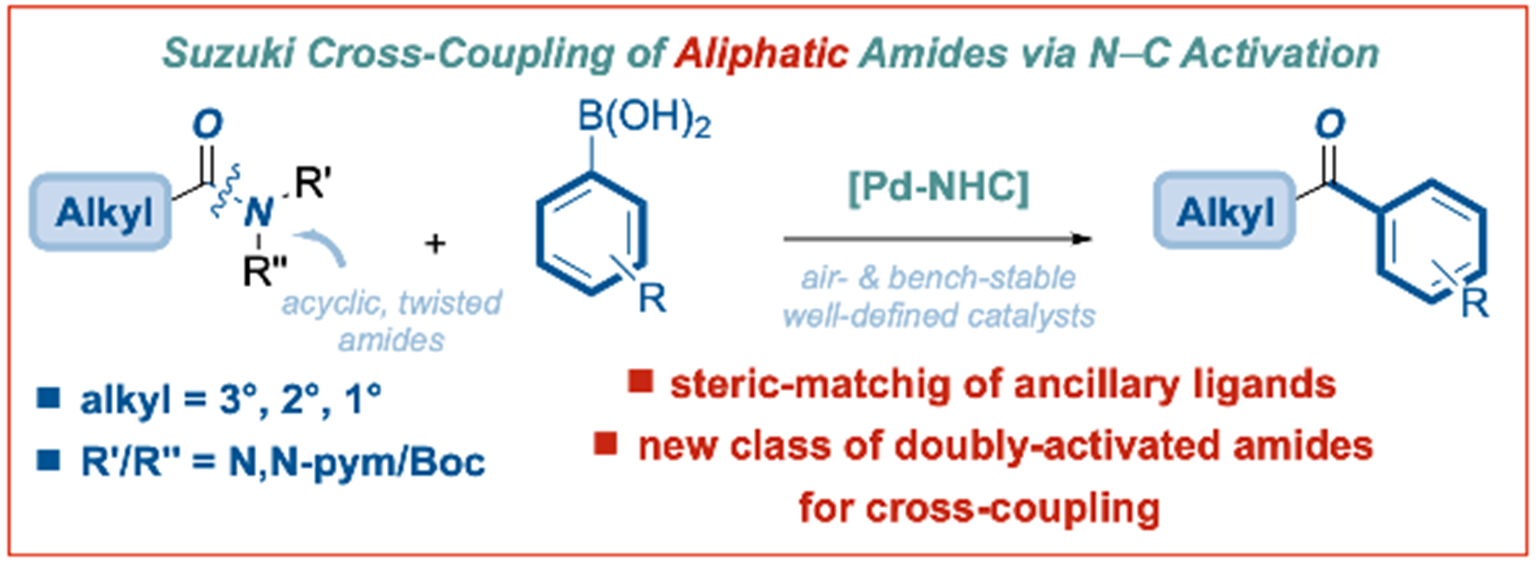
24) Palladium-NHC (NHC = N-heterocyclic carbene)-catalyzed Suzuki–Miyaura cross-coupling of alkyl amides
C. Wang, M. M. Rahman, E. Bisz, B. Dziuk, R. Szostak, M. Szostak*
ACS Catal. 2022, 12, 2426-2433
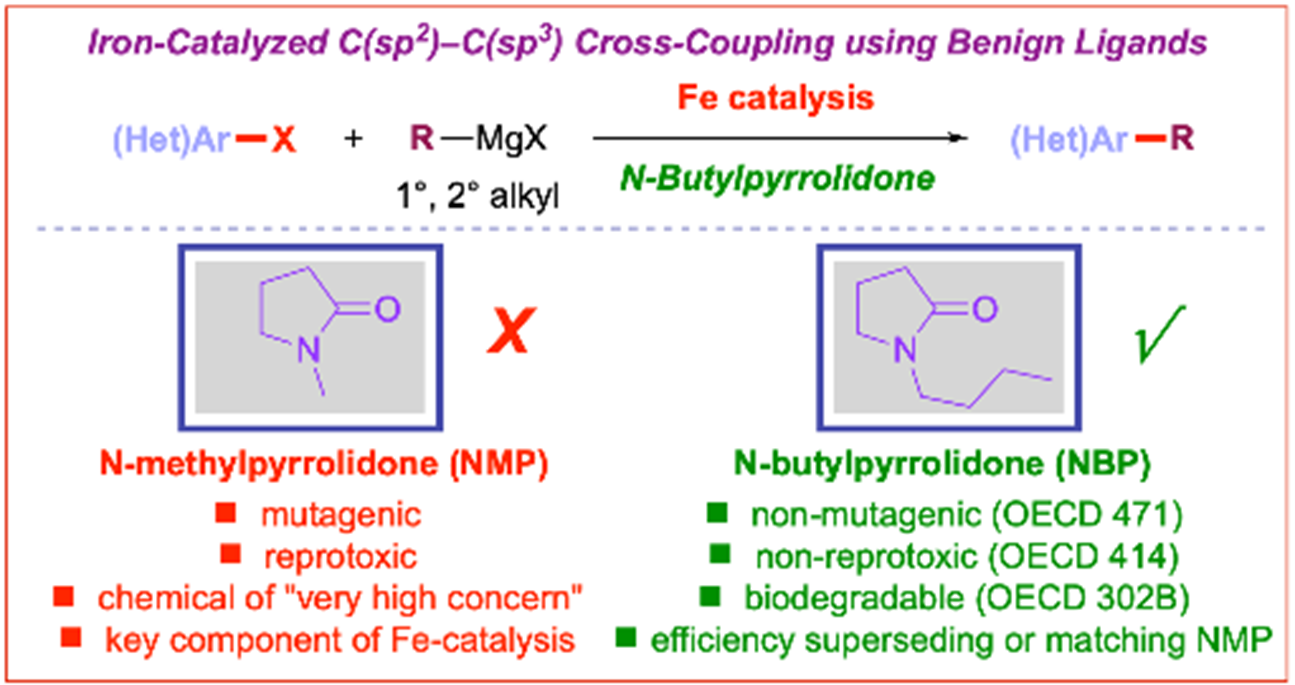
23) N-Butylpyrrolidone (NBP) as a non-toxic substitute for NMP in iron-catalyzed C(sp2)–C(sp3) cross-coupling of aryl chlorides
E. Bisz*, M. Koston, M. Szostak*
Green Chem. 2021, 23, 7515-7521
(Selected as a Hot Article)
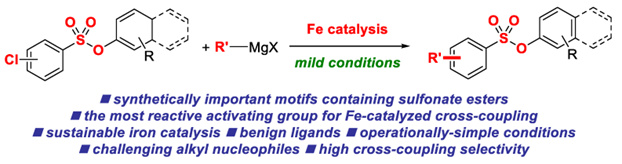
22) Iron-catalyzed cross-coupling reactions of alkyl Grignards with aryl chlorobenzenesulfonates
E. Bisz*
Molecules 2021, 26, 5895-5909
(Special Issue: Ligands in Catalysis; Open-access: https://doi.org/10.3390/molecules26195895)
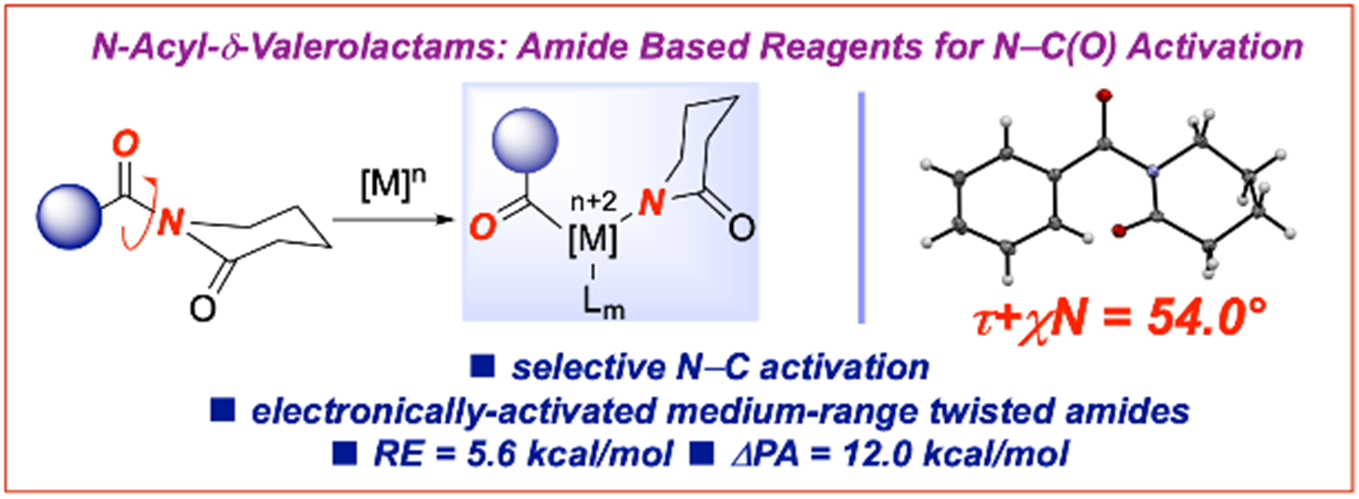
21) Evaluation of cyclic amides as activating groups in N-C bond cross-coupling: Discovery of N-Acyl-d-valerolactams as effective twisted amide precursors for cross-coupling reactions
M. M. Rahman, D. J. Pyle, E. Bisz, B. Dziuk, K. Ejsmont, R. Lalancette, Q. Wang, H. Chen, R. Szostak, M. Szostak*
J. Org. Chem. 2021, 86, 10455-10466
20) Cobalt−NHC catalyzed C(sp2)−C(sp3) and C(sp2)−C(sp2) Kumada cross-coupling of aryl tosylates with alkyl and aryl Grignard reagents
A. Piontek, W. Ochędzan-Siodłak, E. Bisz, M. Szostak*
ChemCatChem 2021, 13, 202-206

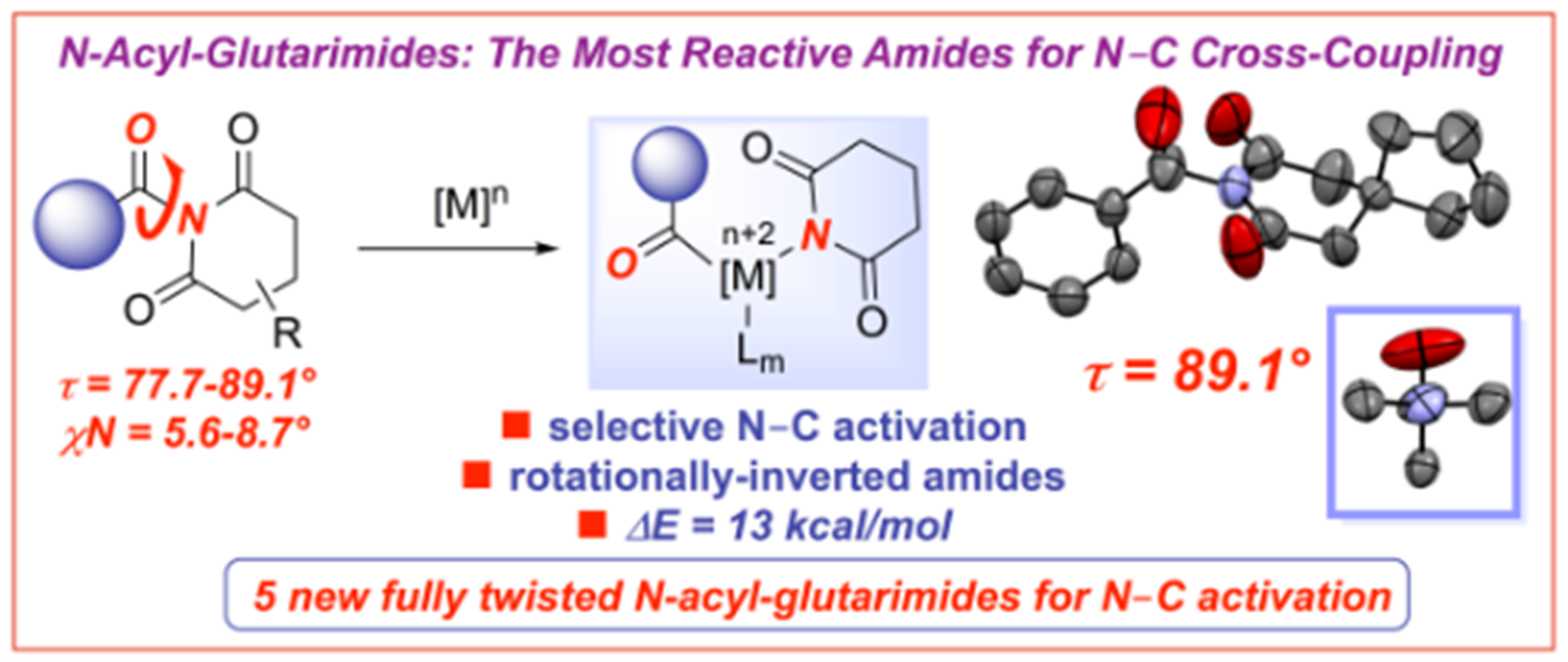
19) N-Acyl-glutarimides: Effect of glutarimide ring on the structures of fully perpendicular twisted amides and N-C bond cross-coupling
M. M. Rahman, C. Liu, E. Bisz, B. Dziuk, R. Lalancette, Q. Wang, H. Chen, R. Szostak, M. Szostak*
J. Org. Chem. 2020, 85, 5475-5485
(Selected as ACS Editors’ Choice: Open-access: https://pubs.acs.org/doi/10.1021/acs.joc.0c00227)
18) Iron-catalyzed C(sp2)–C(sp3) cross-coupling of aryl chlorobenzoates with alkyl Grignard reagents
E. Bisz*, M. Szostak*
Molecules 2020, 25, 230-242
(Special Issue: Recent Advances in Iron Catalysis; Open-access: https://doi.org/10.3390/molecules25010230)
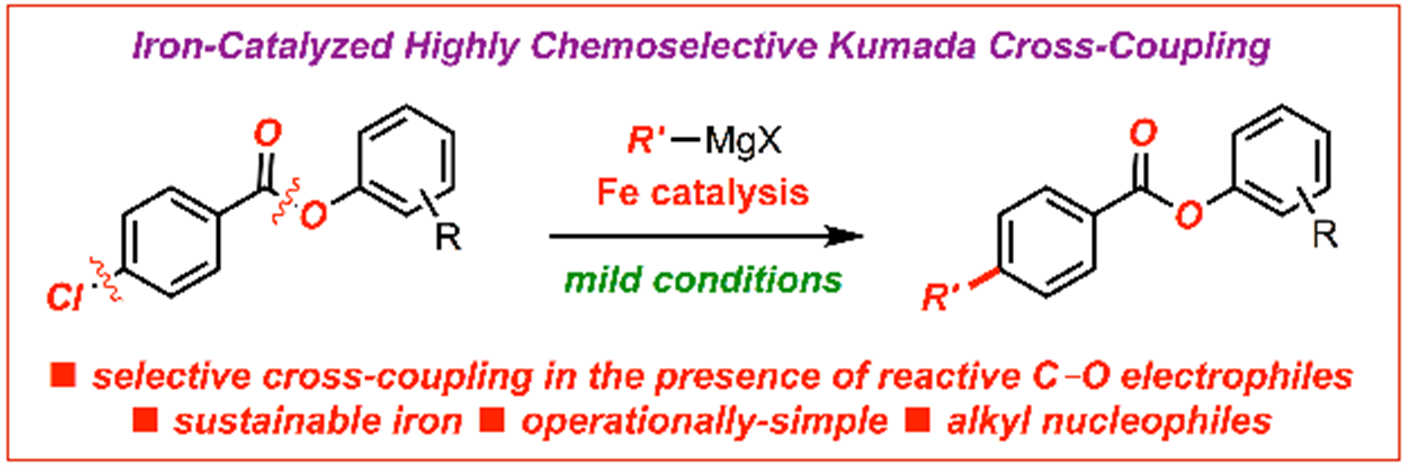
17) Ligand effect on iron-catalyzed cross-coupling reactions: Evaluation of amides as O-coordinating ligands
E. Bisz*, M. Kardela, M. Szostak*
ChemCatChem 2019, 11, 5733-5737
(Special Issue on Sustainable Chemistry to Meet Future Challenges in the Pharmaceutical Industry),
(Selected as a Hot Topic: C-C Coupling)
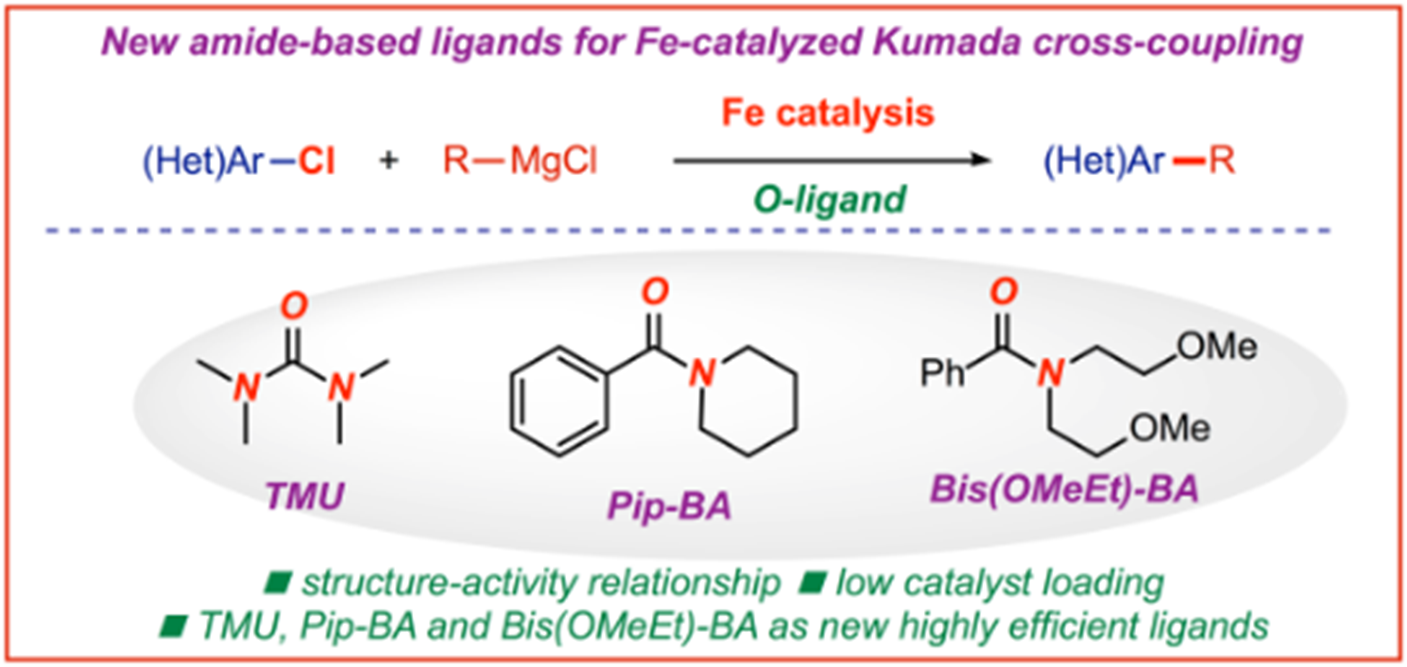
16) Nickel-catalyzed C(sp2)−C(sp3) Kumada cross-coupling of aryl tosylates with alkyl Grignard reagents
A. Piontek, W. Ochędzan-Siodłak, E. Bisz, M. Szostak*
Adv. Synth. Catal. 2019, 361, 2329-2336
(Selected as a Hot Topic: C-C Coupling)
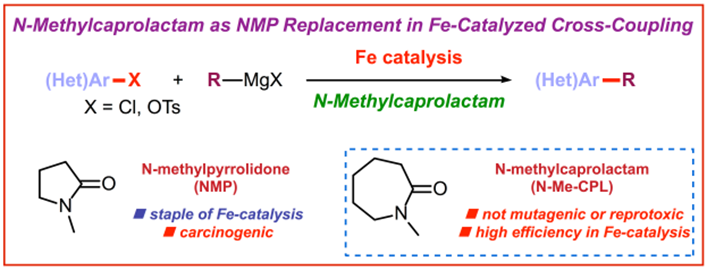
15) N-Methylcaprolactam as a dipolar aprotic solvent for iron-catalyzed cross-coupling reactions: Matching efficiency with safer reaction media
E. Bisz*, P. Podchorodecka, M. Szostak*
ChemCatChem 2019, 11, 1196-1199
(Selected as a Hot Topic: C-C Coupling)
14) Iron-catalyzed C(sp2)–C(sp3) cross-coupling of chlorobenzenesulfonamides with alkyl Grignard reagents: Entry to alkylated aromatics
E. Bisz*, M. Szostak*
J. Org. Chem. 2019, 84, 1640-1646

13) Iron‐catalyzed C(sp2)–C(sp3) cross‐coupling of chlorobenzamides with alkyl Grignard reagents: Development of catalyst system, synthetic scope and application
E. Bisz, M. Szostak*
Adv. Synth. Catal. 2019, 361, 85-95
(Selected as a Hot Topic: C-C Coupling)
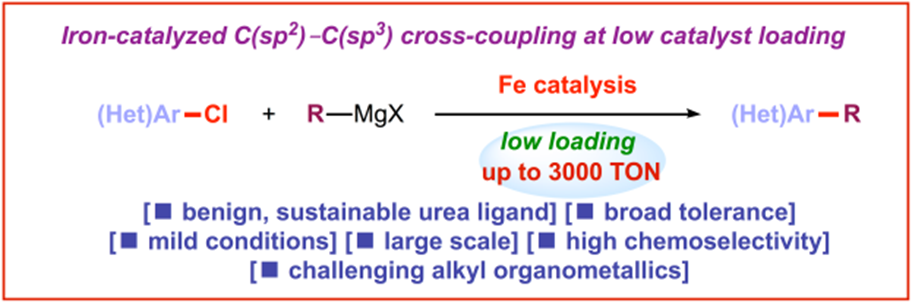
12) Iron-catalyzed C(sp2)–C(sp3) cross-coupling at low catalyst loading
E. Bisz*, M. Kardela, A. Piontek, M. Szostak*
Cat. Sci. Tech. 2019, 9, 1092-1097
11) Structures and energetic properties of 4-halobenzamides
Piontek, E. Bisz, B. Dziuk, R. Szostak, M. Szostak*
Acta Crystallogr. C: Struct. Chem. 2018, 74, 1395-1402

10) Iron-catalyzed cross-couplings in the synthesis of pharmaceuticals: In pursuit of sustainability
A. Piontek, E. Bisz, M. Szostak*
Angew. Chem. Int. Ed. 2018, 57, 11116-11128
(Minireview, Selected as a Hot Topic: C-C Coupling)
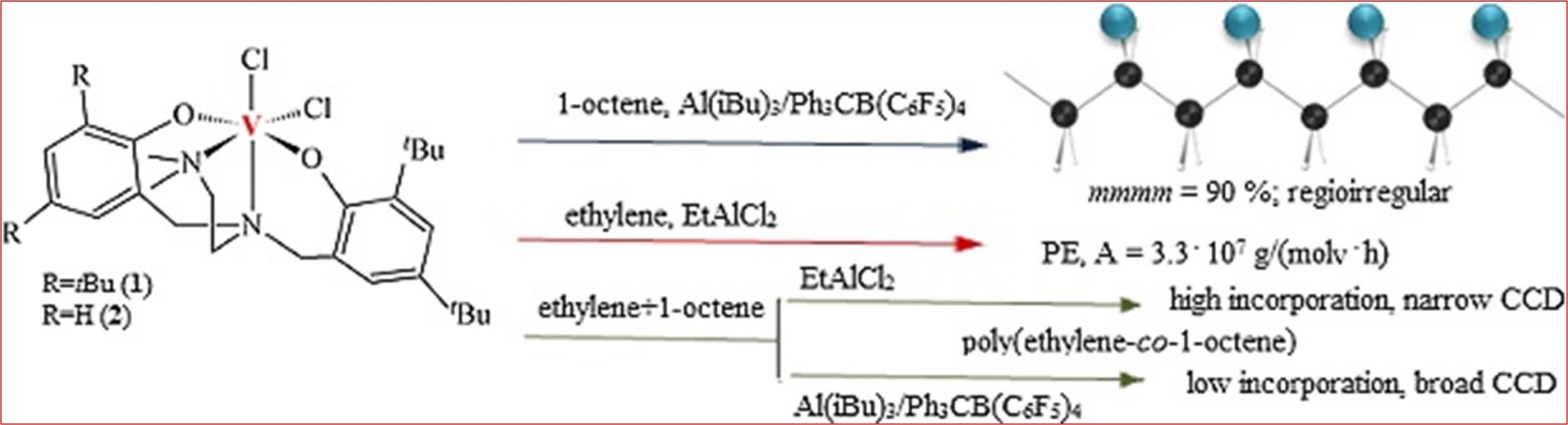
9) Dichlorovanadium(IV) diamine-bis(phenolate) complexes for ethylene (co)polymerization and 1-olefin isospecific polymerization
M. Białek*, E. Bisz
J. Catal. 2018, 362, 65-73
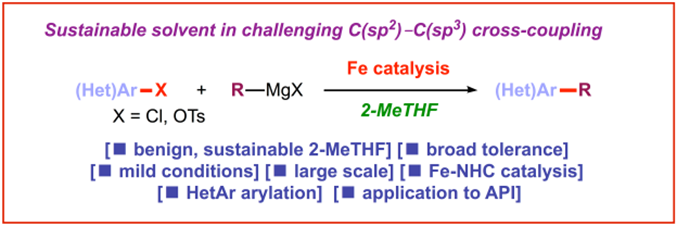
8) 2‐Methyltetrahydrofuran: A green solvent for iron‐catalyzed cross‐coupling reactions
E. Bisz, M. Szostak*
ChemSusChem 2018, 11, 1290-1294
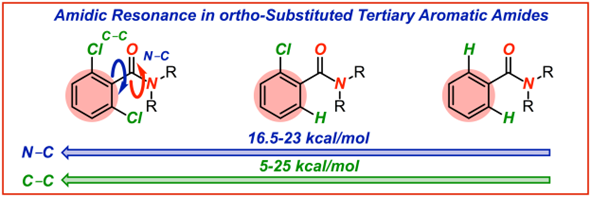
7) Barriers to rotation in ortho-substituted tertiary aromatic amides: Effect of chloro-substitution on resonance and distortion
E. Bisz, A. Piontek, B. Dziuk, R. Szostak, M. Szostak*
J. Org. Chem. 2018, 83, 3159-3163
6) Iron-catalyzed C-O bond activation: Opportunity for sustainable catalysis
E. Bisz, M. Szostak*
ChemSusChem 2017, 10, 3964-3981
(Minireview Article, Selected as Front Cover, Selected for Cover Profile)

5) Polypropylene and poly(ethylene-co-1-octene) effective synthesis with diamine-bis(phenolate) complexes: Effect of complex structure on catalyst activity and product microstructure
M. Białek*, E. Bisz
J. Polym. Sci. A: Polym. Chem. 2017, 55, 2467-2476
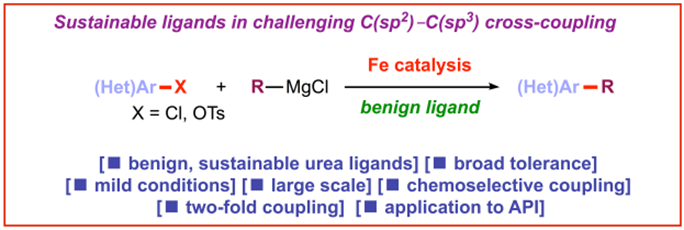
4) Cyclic ureas (DMI, DMPU) as efficient, sustainable ligands in iron-catalyzed C(sp2)–C(sp3) coupling of aryl chlorides and tosylates
E. Bisz, M. Szostak*
Green Chem. 2017, 19, 5361-5366
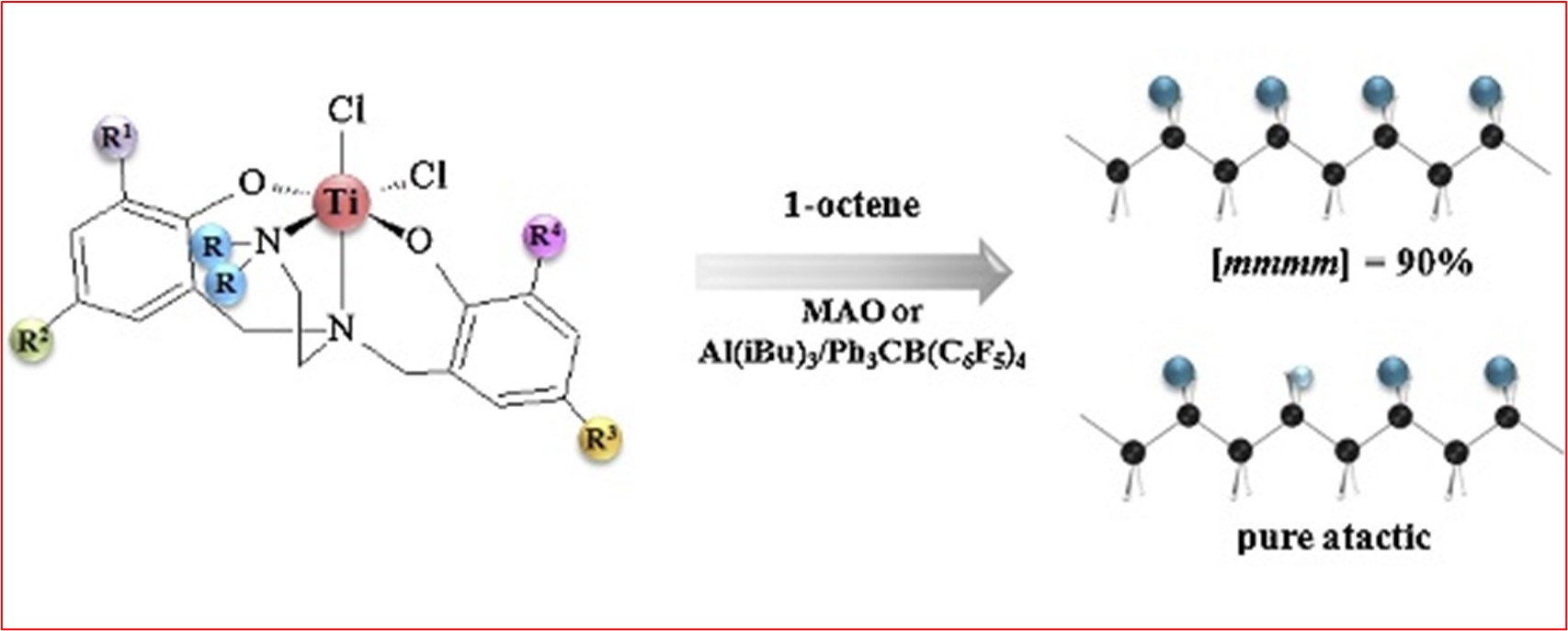
3) Novel diamine-bis(phenolate) Ti(IV) complexes – tuning the complex structure to control catalytic properties in α-olefin polymerization
M. Białek*, E. Bisz
Appl. Catal. A: Gen. 2016, 525, 137-144
E. Bisz, M. Białek*, B. Zarychta
Appl. Catal. A: Gen. 2015, 503, 26-33

1) A comparative study on the polymerization of 1-octene promoted by vanadium and titanium complexes supported by phenoxyimine and salen type ligands
M. Białek*, E. Bisz
J. Polym. Res. 2013, 20, 164-173
